Abstract
EZH2, the methyltransferase subunit of Polycomb Repressive Complex 2 (PRC2), catalyzes H3K27me3 histone modifications and epigenetically regulates genes involved in cellular pluripotency and differentiation. EZH2 plays a role in myeloma (MM) cell proliferation, survival, stemness and its elevated expression correlates with poor prognosis in MM patients. We have previously shown that EZH2 plays a critical role in preventing osteoblast differentiation of myeloma-exposed bone marrow stromal cells (BMSCs). Here we show that GSK126 blocks MM-induced hyperactivation of osteoclast precursors (OCLp). RNA-seq profiling revealed that inhibition of EZH2 prevented RANKL-induced repression of genes associated with bivalent and/or H3K27me3 promoter signatures including OCL inhibitory factors MafB, Irf8, Bcl6b and Arg1. In contrast, we found that OCLp expansion in MM1.S-conditioned media induced significant gene expression changes, which correlated with TNF and IKK signaling, inflammatory responses and CXC-chemokine receptor pathways.
Several classes of small molecule EZH2 inhibitors exhibited anti-MM effects, but their efficacy has been primarily studied in 2-dimensional (2D) cell culture systems or subcutaneous MM-tumor models in vivo. Therefore, we evaluated the effectiveness of GSK126 in the context of the bone microenvironment in a novel 3D model of MM co-cultures (3D-MM). We combined basement membrane extract (BME) hydrogels with devitalized bone slices to mimic the 3D setting of MM with the OCL-resorbing endosteal surface. This enabled us to test GSK126 alone or in combination with bortezomib simultaneously on MM survival and OCLp differentiation and resorption. Differentiating OCLp did not protect MM cells from GSK126 anti-MM effects, nor did the MM cells prevent GSK126 from blocking OCL differentiation. However, mature OCL added to 3D-MM co-cultures increased the IC50 MM inhibition dose of both bortezomib and GSK126 by 40% and 50%, respectively. Further their synergy on MM cells was reduced by 70%.
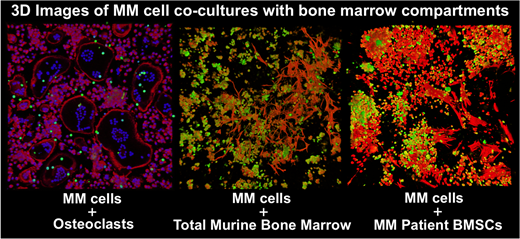
3D-MM co-cultures with total bone marrows harvested from mice of different ages (1-12 months old) showed selective protection from GSK126, but not bortezomib, on MM viability. Furthermore, the resistance to GSK126 was age-dependent. Cultures of bone marrows from older mice exhibited enhanced protection of MM cells from GSK126 as compared to younger marrows. Using confocal microscopy, we found that in addition to soluble factors, physical interaction between MM and bone marrow cells reduced the effectiveness of the epigenetic drug targeting against MM. Depletion of the BMSC population from the total bone marrows using CD45+ selection before establishing the 3D MM co-cultures resulted in diminished protection of MM cell survival from GSK126. Consistent with this, addition of both primary murine and MM-patient derived BMSCs to MM cultures significantly protected MM cells from EZH2 inhibition. In addition to cell-cell contacts, the pro-survival factor IL6 released by mature OCL and BMSCs, has been implicated in mediating chemo-resistance of MM cells. In agreement with this, addition of soluble IL6 to MM-3D cultures significantly protected MM cells from GSK126 inhibition.
Here we show that various cell compartments of the bone microenvironment exhibit differential and drug-specific protection for MM cells from EZH2 inhibition. In addition to direct bone marrow-MM cell interactions, soluble IL6 also exhibits resistance to GSK126. Our novel 3D-MM system enables us to rapidly screen drug combinations, and simultaneously evaluate the influence of bone-microenvironmental interactions on MM drug resistance and bone marrow cell responses to the drugs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal